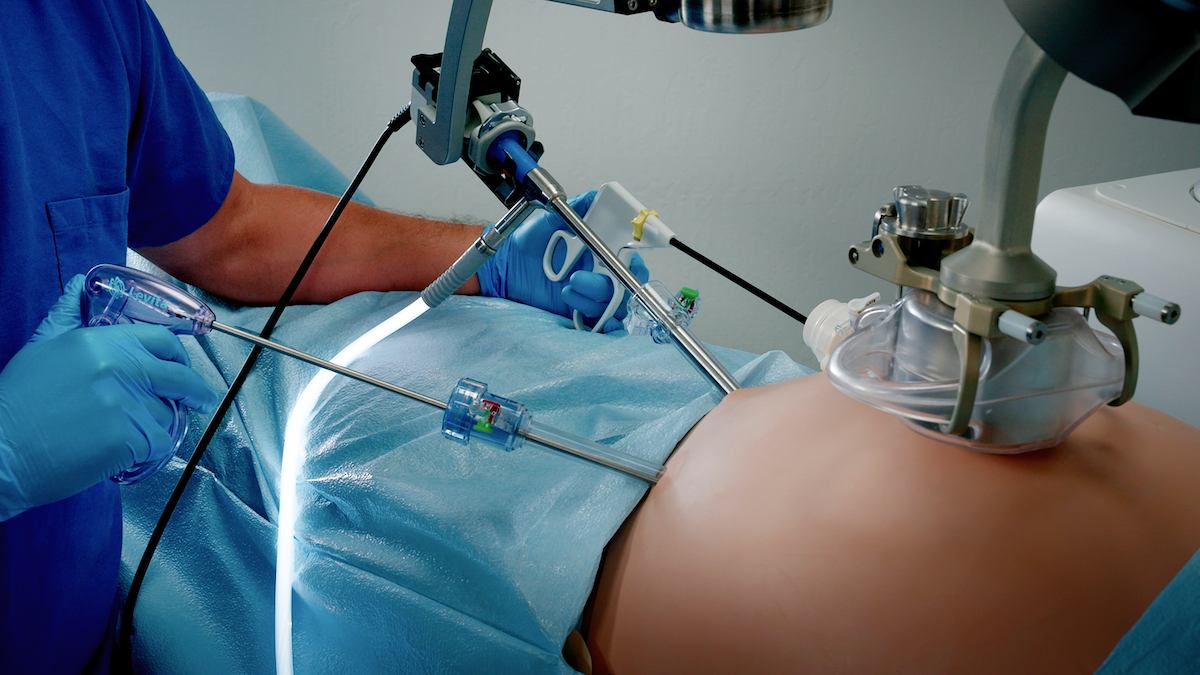
Revolutionary minimally invasive surgery platform designed to benefit patients and medical professionals launched in world-renowned hospital
MOUNTAIN VIEW, Calif., Oct. 24, 2023 /PRNewswire/ — Levita Magnetics, whose mission is to help more patients get access to better surgery, announced today the world’s first commercial use of its MARS™ platform.
Prof. Dr. Matthew Kroh and Dr. Andrew Strong at the Cleveland Clinic performed the first procedures with the new system, cementing a milestone in the advancement of minimally invasive surgery.
The first commercial use, which took place in September, comes just over a month after the Levita MARS system received clearance from the U.S. Food and Drug Administration (FDA).
MARS, a game-changing surgical platform that leverages Levita’s proprietary Dynamic Magnetic Positioning™ technology, had recently gained FDA clearance for use in high-volume abdominal surgeries, including laparoscopic bariatric surgeries, cholecystectomy (gallbladder), prostate and colorectal procedures.
“We’re thrilled to have initiated successful commercial procedures utilizing the MARS system so soon after receiving FDA clearance. We are at the leading edge of a significant shift in the approach to high-volume minimally-invasive abdominal surgery in the United States and in the world,” said Dr. Alberto Rodriguez-Navarro, surgeon, founder, and CEO of Levita Magnetics. “We aim to show that this revolutionary technology can empower surgeons with increased control and better visualization while reducing the number of incisions, delivering significant patient benefits. The MARS system can play a major role in the transition of high volume abdominal procedures to an ambulatory or same day discharge setting.”
MARS builds on the clinical benefits of the Levita Magnetic Surgical System®—faster recovery, less pain, and fewer scars—with surgeon-controlled arms that provide the physician full control over the laparoscopic view and the magnetic retractor, reducing the need for an additional assistant. The console-free system with a compact footprint benefits hospitals and ambulatory surgical centers through increased efficiency, more effective deployment of personnel, and the ability to offer a differentiated patient experience.
“The Levita MARS system featuring Magnetic Surgery has the potential to play a pivotal role in reshaping the landscape of abdominal procedures,” said Prof Dr. Matthew Kroh, MD, Vice Chair of Innovation and Technology at the Cleveland Clinic. “MARS represents a noteworthy advancement and is part of the pathway to enhance minimally invasive surgery for healthcare institutions, surgeons, and most importantly, for patients. It’s also remarkably easy to use.”
Levita remains the sole company that holds an FDA-cleared medical device leveraging the power of magnets to reduce incisions and having a less invasive surgery, delivering a triple impact that benefits patients, surgeons, and hospitals alike. For more information on Levita Magnetics and MARS please visit levita.com.
About Levita
Headquartered in Silicon Valley, Levita Magnetics was founded by innovator and surgeon Dr. Alberto Rodriguez-Navarro. Its proprietary technology is designed to advance minimally invasive surgery. Levita developed the Levita Magnetic Surgical System and MARS, proprietary technologies designed to minimize the footprint of surgery and improve patient outcomes. For more information visit levita.com.